Sodium Dichloroisocyanurate and Trichloroisocyanuric Acid: Properties and Applications
Sodium dichloroisocyanurate (NaDCC) and trichloroisocyanuric acid (TCCA) are chlorine-based disinfectants used in water treatment. TCCA provides long-term disinfection, while NaDCC dissolves quickly for rapid sanitation. Both contain cyanuric acid (CYA) as a stabilizer, preventing chlorine loss under UV exposure. Proper CYA management ensures effective and balanced disinfection.

1. Introduction
Sodium dichloroisocyanurate (NaDCC) and trichloroisocyanuric acid (TCCA) are widely used chlorine-based disinfectants in water treatment, swimming pool sanitation, industrial sterilization, and other hygiene-related applications. Both compounds have high available chlorine content and release hypochlorous acid (HOCl) in water, effectively eliminating bacteria and viruses. Additionally, they contain cyanuric acid (CYA) structures, which help stabilize chlorine in outdoor conditions by reducing ultraviolet (UV) degradation. This article explores their synthesis, decomposition mechanisms, chlorine content calculation, stabilizer functions, and their applications in swimming pool disinfection.
2. Synthesis of TCCA and NaDCC
(1) Trichloroisocyanuric Acid (TCCA)
TCCA is synthesized from cyanuric acid (C₃H₃N₃O₃) through a chlorination reaction:
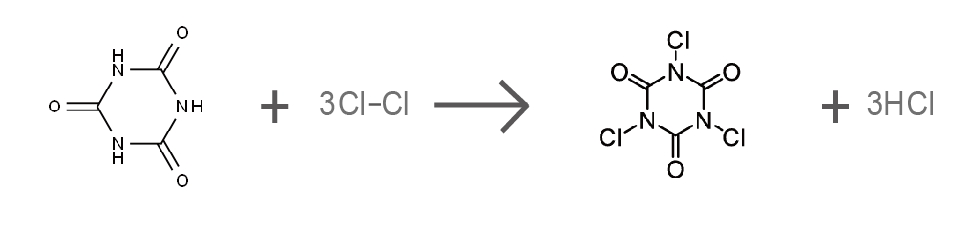
TCCA contains three chlorine atoms that can be released in aqueous solutions, giving it a high available chlorine content of approximately 90%.
(2) Sodium Dichloroisocyanurate (NaDCC)
NaDCC is synthesized similarly but with a lower degree of chlorination and the introduction of sodium ions:
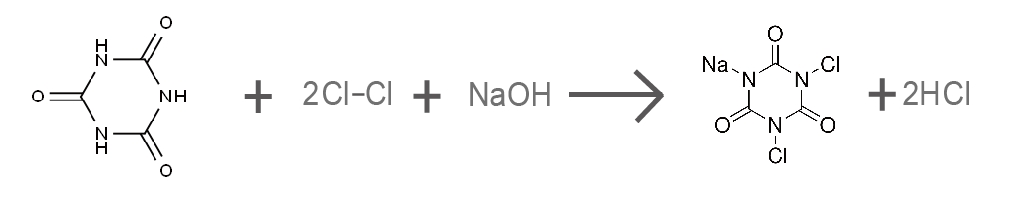
Since NaDCC releases only two chlorine atoms, its available chlorine content is relatively lower (about 56-60%).
3. Decomposition in Water and Chlorine Release Mechanism
(1) Chemical Reactions
When dissolved in water, TCCA and NaDCC undergo hydrolysis, releasing hypochlorous acid (HOCl), the primary disinfectant:
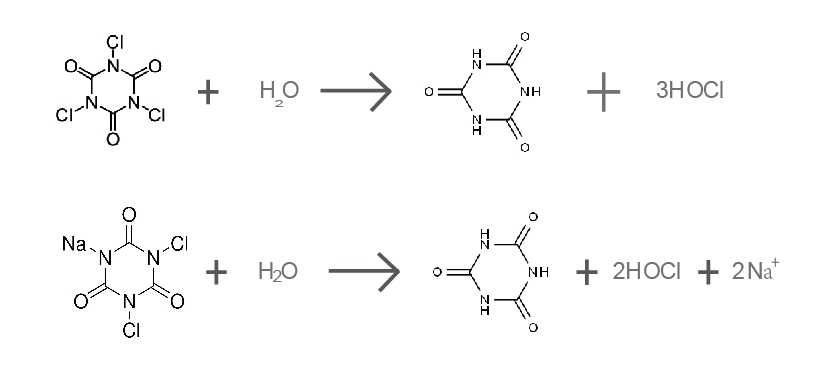
TCCA releases three HOCl molecules, whereas NaDCC releases only two. TCCA has stronger disinfection capabilities, while NaDCC dissolves faster, making it suitable for rapid sanitation applications.
(2) Reversibility and Equilibrium Control
This hydrolysis process is reversible. When chlorine levels decrease (e.g., due to HOCl consumption), the reaction shifts to the right, releasing more chlorine. Conversely, when excess HOCl is present, the reaction reverses, slowing down chlorine release. Under proper conditions, both TCCA and NaDCC provide a sustained release of chlorine, maintaining effective disinfection over time.
4. Calculation of Available Chlorine Content
Available chlorine refers to the proportion of chlorine in a compound that can be released as an active disinfectant. It is calculated as follows:
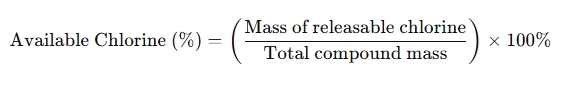
For TCCA:
- Molecular weight: 232.41 g/mol
- Mass contribution of 3 chlorine atoms: 106.35 g
- Calculation:
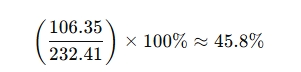
- Since one molecule of TCCA releases three active chlorine atoms, its available chlorine content is approximately 90%.
NaDCC follows a similar calculation, with an available chlorine content of 56-60%.
5. Cyanuric Acid as a Stabilizer
(1) The Role of Cyanuric Acid in Stabilizing Chlorine
In swimming pool sanitation, chlorine degrades rapidly under UV exposure. Cyanuric acid (CYA) acts as a stabilizer by forming a HOCl-CYA complex, which reduces the rate of chlorine degradation:
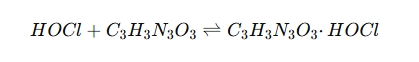
This complex slows down chlorine loss, ensuring a longer-lasting disinfectant effect.
(2) Do Market-Available Chlorine Tablets Contain Cyanuric Acid?
Since TCCA and NaDCC naturally contain cyanuric acid groups, commercially available chlorine tablets typically do not require additional CYA. However, for other chlorine-based disinfectants like sodium hypochlorite (NaClO), cyanuric acid is often added separately to enhance stability.
(3) Control of Cyanuric Acid Levels
- Optimal concentration: 30-50 ppm (higher levels reduce disinfection efficiency).
- Excess CYA can lower chlorine activity, as excessive binding reduces free available chlorine.
- If CYA exceeds 100 ppm, dilution with fresh water is necessary to restore effectiveness.
6. Comparison and Selection of TCCA and NaDCC
| Feature | Trichloroisocyanuric Acid (TCCA) | Sodium Dichloroisocyanurate (NaDCC) |
|---|---|---|
| Available Chlorine Content | 90% | 56-60% |
| Dissolution Rate | Slow | Fast |
| pH Effect | Slightly lowers pH | Slightly raises pH |
| Best Applications | Long-term disinfection, continuous dosing | Rapid sanitation, emergency disinfection |
| Stabilizer Content | Contains cyanuric acid for stability | Contains cyanuric acid but degrades faster |
(1) TCCA is ideal for:
- Swimming pools, industrial water treatment, and cooling towers where long-term chlorine release is required.
- Slow-release applications, maintaining chlorine levels over time.
(2) NaDCC is ideal for:
- Emergency disinfection (e.g., drinking water treatment, hospital sanitation).
- Situations requiring rapid dissolution and immediate chlorine action.
7. Conclusion
Sodium dichloroisocyanurate (NaDCC) and trichloroisocyanuric acid (TCCA) are two highly effective chlorine-based disinfectants that release hypochlorous acid (HOCl) in water. Both compounds contain cyanuric acid structures, which help stabilize chlorine and prolong its disinfecting action. TCCA has a higher chlorine content and is suitable for long-term water treatment, while NaDCC dissolves quickly and is ideal for rapid disinfection. Proper use of cyanuric acid, maintaining its concentration within an optimal range, can maximize chlorine efficiency and ensure safe, clean water. Choosing the right chlorine disinfectant based on application needs can lead to more efficient and cost-effective sanitation management.